
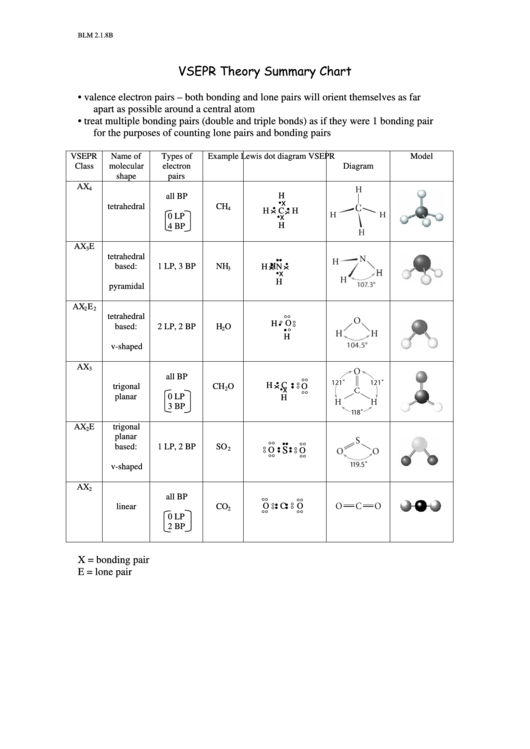
Browse more Topics under Chemical Bonding And Molecular Structure It is useful for nearly all compounds that have a central atom that is not a metal. Specifically, VSEPR models look at the bonding and molecular geometry of organic molecules and polyatomic ions. It is basically a model to predict the geometry of molecules. As you can see, there are three blue bubbles of substituents and no lone pairs, meaning the VSEPR notation at this specific carbon is AX 3, meaning it will be trigonal planar.The Valence Shell Electron Pair Repulsion Model is often abbreviated as VSEPR (pronounced “vesper”). Each blue bubble represents a different substituent group (or atom) coming off of that carbon. We can do the same thing for the carbon second from the right, as shown in the image above. VSEPR predicts this will be a tetrahedral carbon atom as it has the AX 4 configuration of four bonded groups and no lone pairs, as we treat each hydrogen atom as a separate substituent and the everything else residing to the right of the carbon as one substituent. In the example above, we will only examine the carbon furthest to the left. One way you can use VSEPR is to call a group of atoms one substituent. Q: Does VSEPR theory work for more complex molecules?Ī: Yes, it can, however, it is important to remember that VSEPR is a tool and has its limits. So….what we need to remember is that for the AX 5 group, you need to replace equatorial atoms with lone pairs AND for the AX 6 group, you need to replace the atoms on the axis with lone pairs, as we have shown above. Thus, we can’t just substitute a lone pair for any old atom. Here, there is a geometric difference between the atoms on the axis (called axial substituents) and the ones around the middle, called the equatorial substituents. It get a little trickier when we get to the 5 and 6 substituent molecules (AX 5 group and AX 6 group, respectively). Same for AX 3E because all of the atoms are geometrically equivalent. So for AX 2E, it is simple to see that we get trigonal pyramidal as the answer because we can replace any of the atoms with a lone pair because they are all geometrically equivalent. In the chart above we have tried to show how this works by just blurring out an atom for a lone pair.įor the 3 and 4 substituent molecules (AX 3 group and AX 4 group, respectively) it is easy to do this because each one of the substituent atoms is the same. Here is one way to remember this chart: Think about each lone pair as just replacing an atom. We know this because of the bond angles associated with each of the four types of shapes. Hence, simple molecules (like the ones we are looking) at will tend to place substituent atoms as far from each other as possible. We also know that electrons repel each other. Let’s not forget, the whole purpose of VSEPR is to minimize interactions between the substituents (atoms and lone pairs) of a molecule. More about VSEPR and the molecular geometry of NO 2 –: Even though one is a double bond and the other is a single bond, they are actually the same because of resonance, a process where the single and double bond are changing places so rapidly that they act like it is 1.5 bonds between the O and N.Īlso, it is important to remember that there is a negative charge on the oxygen that has only one bond, giving the overall molecule a negative charge. It is the O-N-O bond angle, which is 132 degrees. There is only one bond angle in this molecule. Bond angles help show molecular geometry of NO 2 – As you can see from the chart, AX 2E molecule is bent. Step 3: Use the VSEPR table to determine the geometry of NO 2 –. For the purposes of VSEPR, we are determining the geometry of the nitrogen atom, so we ignore the negative charge on the oxygen. Step 2: Apply the VSEPR notation to the molecule.įor this one, we can see that it has one central atom, two surrounding atoms, and one lone pair of electrons around the central atom, making it AX 2E.
#Vsepr notation how to
Step 1: Determine the Lewis structure of the molecule.įor NO 2 –, it is as shown below: For a full-explanation of how to figure out the Lewis structure, please go to Lewis Structure of NO 2 –. There is an easy three-step process for determining the geometry of molecules with one central atom.
NO 2 – looks like this: How do you find the molecular geometry of NO 2 –? Hence, the molecular geometry of NO 2 – only has 134 degree bond angles in the molecule.
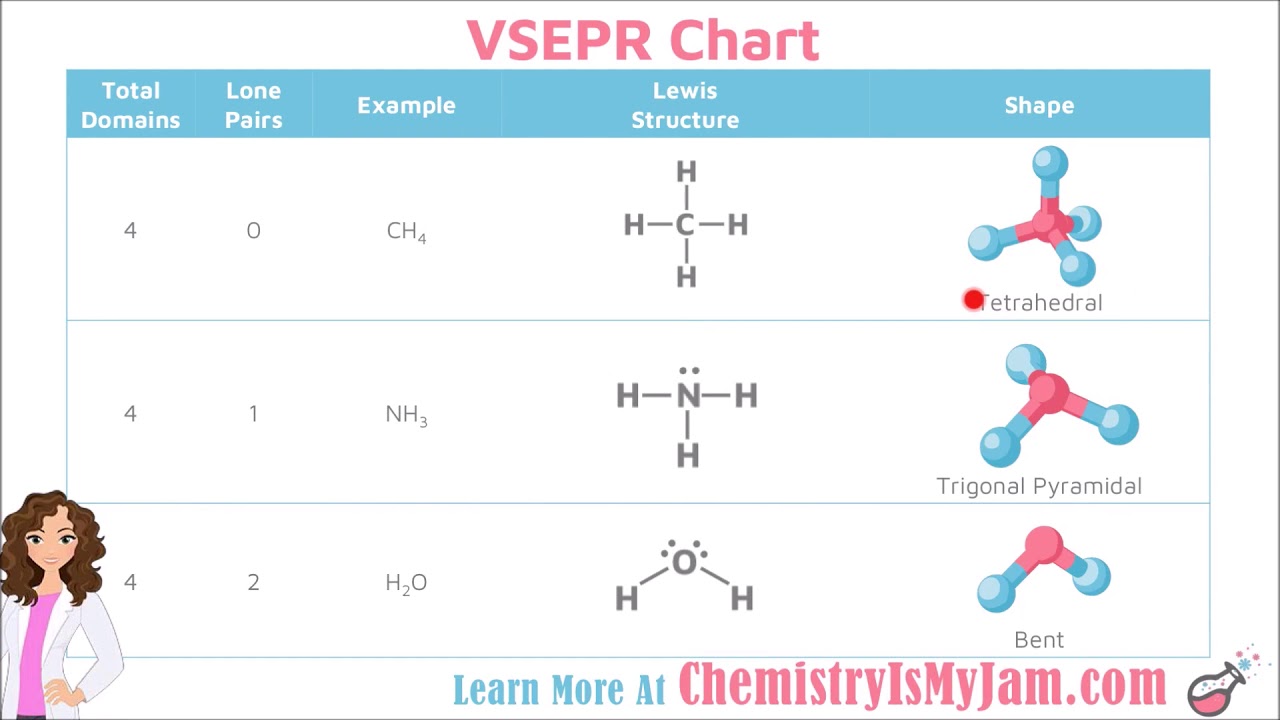
The molecular shape of NO 2 – is bent, or AX 2E using Valence Shell Electron Pair Repulsion (VSEPR) theory. What is the molecular geometry of NO 2 –?


 0 kommentar(er)
0 kommentar(er)
